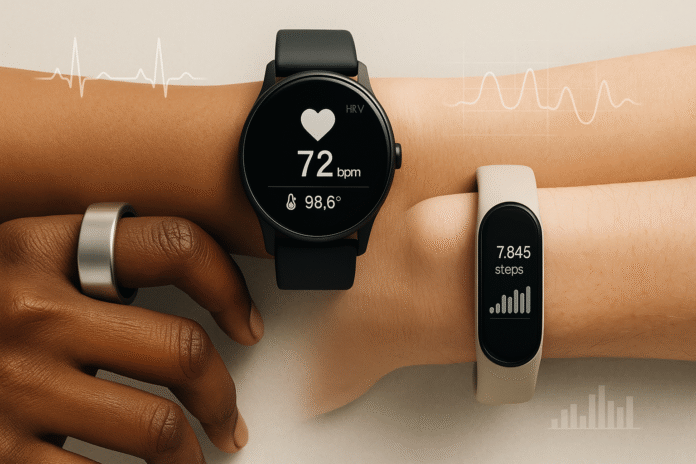
In 2025, the era of ‘AI wearables’ has dawned, ushering in a new wave of health technology. These devices, which continuously monitor physiological signals and utilise machine learning, have transitioned from novelty to necessity.
From rings and watches that can detect heart rhythm irregularities on a population scale to devices that screen for sleep apnea and even continuous glucose monitors for non-insulin-requiring diabetes, the potential of AI wearables is vast and promising.
Meanwhile, women’s health and mental well-being are moving from the edges of fitness tech to the centre: cycle-aware contraception apps can ingest data from rings and watches, hot-flash cooling wristbands are being tested clinically, and stress-aware “AI coaches” are appearing inside the apps you already use.
The promise is substantial: earlier detection, more tailored advice, and day-to-day support in local contexts. The caveats are real: algorithmic bias (e.g., optical sensors on darker skin), privacy trade-offs, noisy data that can mislead, and the risk of drifting from “wellness” into regulated medical territory without the evidence to back it.
This feature maps the terrain — including definitions, what currently works, what remains experimental, and what to watch next — so readers can distinguish between signal and noise.
What we mean by “AI wearable” in 2025
AI wearable: a consumer or medical device worn on the body (e.g., ring, watch, band) that captures physiological signals (such as heart rate, skin temperature, movement, or skin conductance) and uses machine-learning models to generate insights or recommendations.
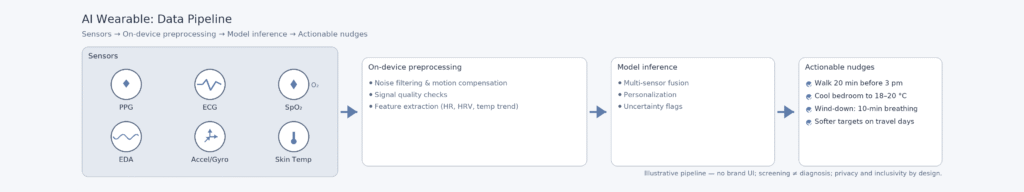
Popular sensors include PPG (photoplethysmography — an optical method to estimate pulse and its variability), ECG (electrocardiogram — an electrical tracing of heart rhythm), SpO₂ (peripheral blood oxygen saturation from reflected light), EDA (electrodermal activity — skin conductance linked to sweat gland activity), accelerometers/gyroscopes for motion, and skin temperature sensors for circadian or cycle-related changes. “AI” in this context encompasses a range from classical pattern recognition to large language models (LLMs) that converse about your data.
Why the definition matters: features with diagnostic or screening claims often cross into medical-device regulation (e.g., ECG, AFib detection, sleep apnea screening), while wellness features (e.g., “stress score”) usually do not — unless a regulator decides they do. The distinctions shape what companies can claim and what evidence is expected.
The 2025 landscape at a glance: rings, watches, and regulated features
Here’s the state of play: consumer devices with a few truly regulated features (ECG, AFib screening, OSA triage) and a long tail of wellness metrics. Use this section to separate proven capability from marketing fog.

Heart rhythm screening on the wrist is now a routine procedure
Apple’s ECG app received a U.S. FDA De Novo classification in 2018 for the single-lead ECG detection of AFib, paving the way for consumer ECG devices to become available. In 2022, Fitbit gained FDA clearance for a passive, PPG-based algorithm that flags irregular rhythms suggestive of AFib during rest and sleep. FDA Access Datablog. Google
Sleep apnea screening is coming to mainstream wearables
Samsung’s Galaxy Watch sleep-apnea feature received U.S. authorisation in 2024 and has since been expanded in some markets; Samsung and Stanford Medicine announced further research in 2025.
These tools detect signs of moderate to severe obstructive sleep apnea over multiple nights and are not a replacement for a clinician’s diagnosis. AASMSamsung Newsroom+1
Smart rings go mainstream.
Samsung’s Galaxy Ring was launched with an explicit “AI-powered” focus on sleep and health, featuring tight integration with Samsung Health. It joins Oura, Ultrahuman, RingConn, and others in the ring category.

Over-the-counter CGM arrives
In 2024, the FDA authorised Dexcom Stelo, the first OTC continuous glucose monitor designed for people not using insulin — a watershed for metabolic tracking beyond diabetes care. Wikipedia
Cuff-based blood pressure monitoring in a watch exists (and cuffless remains a contested concept).
Omron’s HeartGuide utilises a tiny inflatable cuff to take oscillometric readings and has received 510(k) clearance. Cuffless methods (camera/PPG-based) are improving but still face questions regarding accuracy for clinical use.
AI “coaches” reach consumers. Whoop’s Coach utilises OpenAI to answer personalised questions based on your data; Google is rolling out a Gemini-powered coach in the Fitbit app that adapts plans and advice. Reviewers note both potential and the risk of “hallucinations,” so most products ship with disclaimers and guardrails.
Key distinction — screening vs diagnosis: Wearables that screen for conditions (e.g., AFib notifications, OSA signs) nudge you toward a clinician; they do not diagnose. That line keeps expectations realistic and protects users while evidence matures.
What’s actually different now: the AI layer
The leap isn’t new sensors—it’s models that fuse them and explain “why” a metric moved, then suggest the smallest practical action. This is where value—and risk of bad advice—lives.
The pivotal shift in 2025 is the move from raw data to personalised health guidance. Instead of just providing a step count or a sleep score, devices are now capable of:
- Combine multi-sensor inputs (HR, HRV, temp, movement, SpO₂) using machine-learning models,
- Summarise the “why” behind a spike, dip, or pattern (e.g., “jet lag likely; bedtime drifted; HRV suppressed”),
- Convert it into actionable nudges (“walk 20 minutes before 3 pm,” “cool bedroom to 18–20°C,” “take it easy today — strain target 7–9”).
While the potential benefits are clear, it’s important to note that variance in sensor quality, individual physiology, and model training can lead to misleading results.
Independent research consistently underscores that many mental-health and stress detections are promising but not yet clinic-ready, providing a reliable guide for the future of AI wearables.
Women’s health: from fertility awareness to menopause support
Women’s health has shifted from afterthought to design target. Cycle-aware algorithms and menopause-aware comfort tools demonstrate how the same sensors can provide life-stage-specific support.
Cycle-aware birth control and planning
The FDA-cleared Natural Cycles app — a software medical device for contraception — can now ingest wearable temperature data (Oura Ring since 2021, Apple Watch wrist temperature since 2023) to calculate daily fertility status without the need for manual thermometers. This expands access and convenience for hormone-free birth control and planning.
Definition — basal/wrist temperature (BTT): tiny overnight changes (often <0.5°C) that shift with ovulation and cycle phase; wearables estimate trends, not absolute body temperature.
Natural Cycles remains responsible for the contraceptive algorithm and regulatory claim; Oura and Apple supply temperature inputs once a user consents. This division of roles is crucial for ensuring safety and accountability.
Menopause and perimenopause
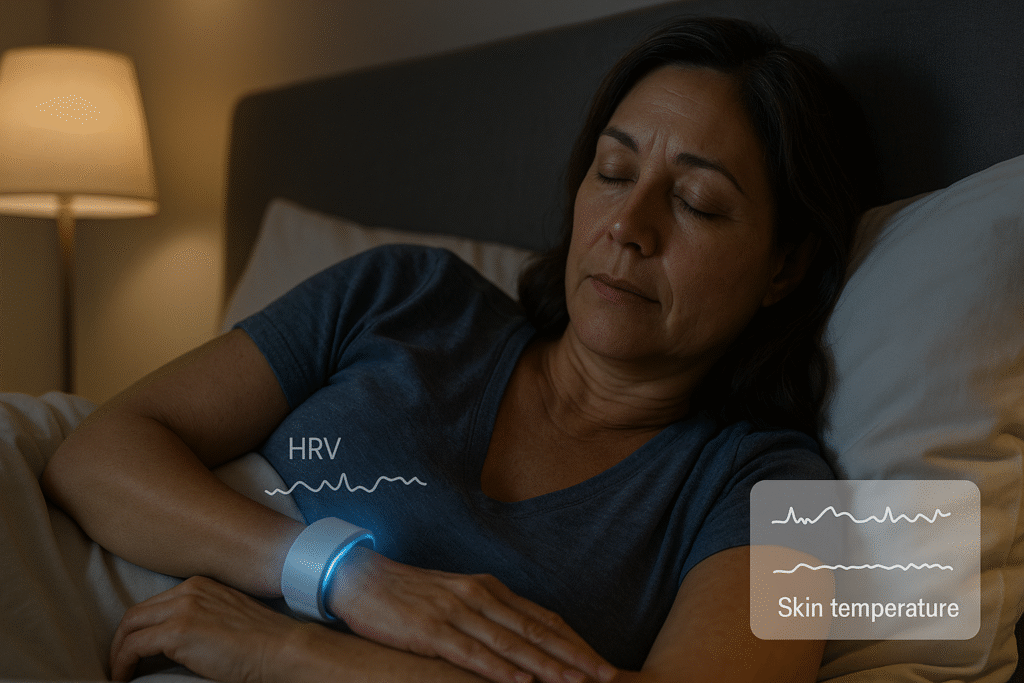
Two trends stand out:
- Rings and watches as “symptom diaries.” Rings like Oura track overnight HRV (heart-rate variability — a measure of beat-to-beat variation tied to autonomic balance), resting heart rate, and skin temperature. Oura reports distinctive pattern changes across the menopausal transition, which are worthwhile for self-awareness and clinician conversations, although not for diagnosis.
- Hot-flash comfort devices enter trials. Embr Wave — a wrist device that delivers targeted cooling or warming — has published feasibility studies showing reductions in sleep disruption and perceived hot-flash interference among menopausal women and cancer survivors; randomised controlled trials are being planned or piloted. Consumer outlets note that the evidence is still early.
Why this matters: In many countries, women’s health has been under-invested in. The shift from “one size fits all” sleep and activity tracking to cycle- and life-stage-aware insights is a genuine step forward — provided devices are transparent about limits and inclusive in design.
Mental health: steady progress, careful claims
What wearables can (and cannot) say in 2025
- Activity and mood: Large studies and meta-analyses link higher daily steps and physical activity with lower depressive symptoms. Wearables help quantify and nudge activity, which is one of the most robust behavioural levers for mood. However, correlation does not imply causation, and individual responses vary.
- Digital phenotyping: Research teams use wearable signals (sleep timing/regularity, HRV, movement patterns) to train models that screen for the probability of depression or anxiety and track relapse risk. Systematic reviews conclude the field is promising yet not ready for routine clinical diagnosis; data sparsity, heterogeneity, and privacy are active challenges.
- Stress sensors: Devices with EDA (e.g., Fitbit Sense/Pixel Watch) and HRV-based stress indices (e.g., Oura, Whoop) estimate “physiological stress” in real time. Journalists and researchers alike note that stress is multi-dimensional: a thrilling match or sauna may elevate “stress” signals without harm; the coaching layer should contextualise and avoid pathologising normal responses.
- Journaling and mood check-ins: Apple’s State of Mind feature on iPhone and Apple Watch allows users to log their mood and see correlations with sleep, daylight, and activity — an evidence-based but straightforward practice that can support help-seeking, especially when paired with standardised screeners.
Generative AI coaches meet mental health.
Whoop’s Coach and Google’s Gemini-based Fitbit coach now answer natural language questions, such as “Why was my HRV low after travel?” Reviewers applaud the potential of LLMs but caution about the risk of hallucinations or persuasive but inaccurate advice, particularly in areas such as mental health or nutrition. Responsible products bind answers to policy and show sources where possible.
Sleep and respiratory health: from “how long” to “how risky”
Traditional sleep staging from accelerometers is old news; the 2025 shift is toward condition-adjacent signals:
- Obstructive Sleep Apnea (OSA) screening on watches: Samsung’s FDA-authorised feature estimates whether your multi-night data show signs consistent with moderate-to-severe OSA. It’s a triage tool: see a clinician for diagnosis.
- Contactless or ring-based options are in development or have niche use, but validated pathways remain watch- or finger-sensor-based, with either FDA authorisation or country-specific licenses.
- Sleep and cardiometabolic links: Observational studies using Fitbit-scale datasets continue to show relationships between sleep duration/consistency, and obesity or sleep apnea risk — providing proper population signals, not personal diagnoses.
Definition — OSA: repeated airway collapse during sleep, causing oxygen drops and micro-arousals; linked to cardiovascular and cognitive risks.
Cardiometabolic health: glucose and blood pressure on your wrist (and finger)
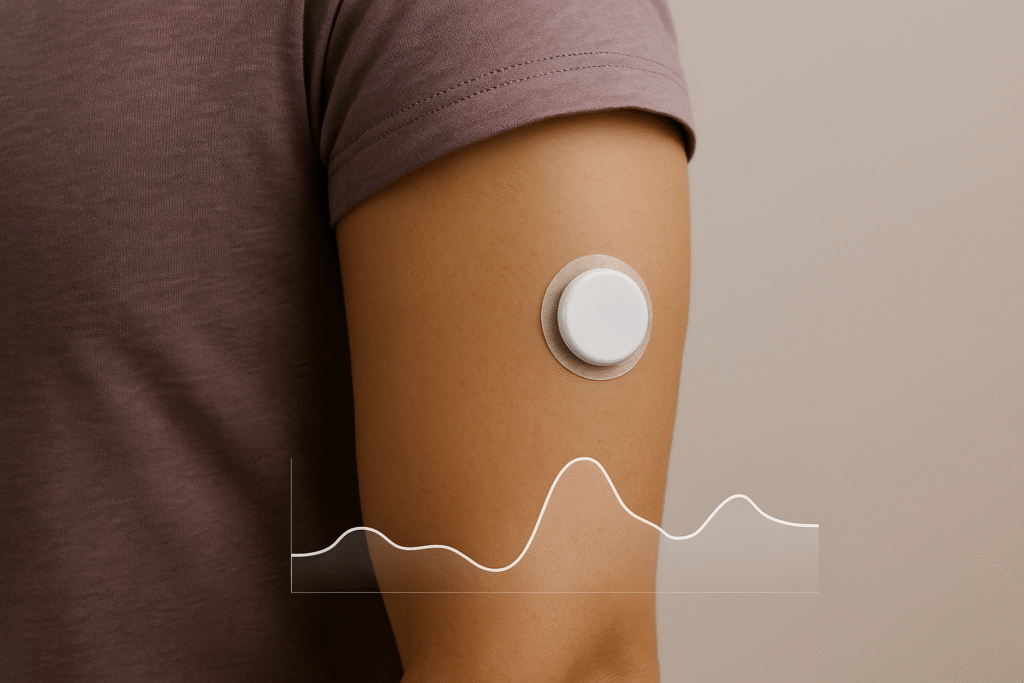
- OTC CGM (Dexcom Stelo): The first over-the-counter CGM clears a path for people managing diet, exercise, or weight — but users should understand intended use and avoid medical decisions without clinician guidance.
- Blood pressure: Only a handful of devices provide validated readings at home. Omron’s HeartGuide uses a mini-cuff; most smartwatches with cuffless BP estimation remain wellness features, with accuracy under active study and debate for clinical adoption. Regulators and hypertension societies urge caution.
Definition — HRV: the variation in time between heartbeats; higher waking HRV is often interpreted as greater parasympathetic (rest-and-digest) tone, though context matters.
Equity and Accuracy: The challenging problems
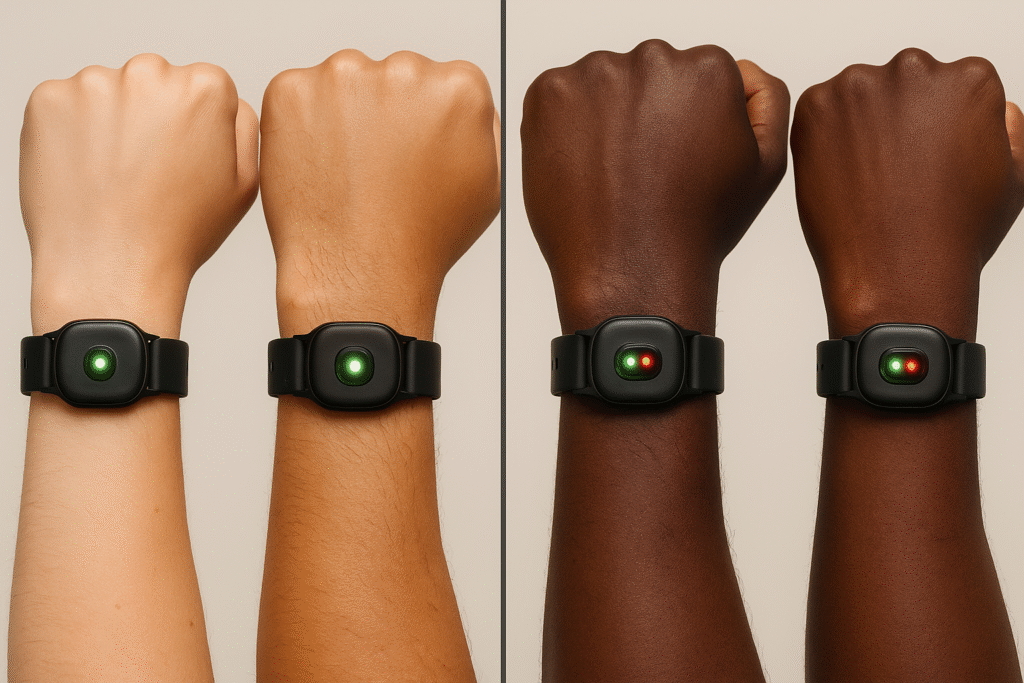
Skin tone bias and optical sensors
Pulse oximetry’s well-documented bias — overestimating oxygen saturation in darker skin — has triggered FDA advisory scrutiny and underscores the need for improved testing across diverse skin tones.
Optical heart-rate sensors (PPG) on consumer devices can also vary due to differences in pigmentation and perfusion. Manufacturers are investing in multi-wavelength designs and algorithms, but independent validation remains essential.
“Cuffless everything” and the validation gap
Cuffless BP, non-invasive glucose from smart rings/watches, and generalised “stress” scores face physics and physiology constraints. The FDA has explicitly warned about smartwatches and rings that claim to measure blood glucose without piercing the skin — these devices are not authorised and may be dangerous.
Data quality and context
Even good sensors produce noisy data when worn loosely, during vigorous motion, or in extreme temperatures. AI models trained on pristine lab data can misread real-world patterns.
Systematic reviews of wearables for depression screening emphasise the quality of datasets, ground-truth labelling, and transparent reporting.
Privacy in practice: what happens to your health data?
- Apple states that Health app data is end-to-end encrypted with iCloud (and unreadable by Apple) when the default protections are in place; Advanced Data Protection extends end-to-end encryption to more categories.
- Google/Fitbit has publicly committed that Fitbit health and wellness data are not used for Google Ads and are kept separate from ad systems; the Fitbit privacy policy outlines these controls. Critics continue to scrutinise implementation details as accounts migrate to Google.
Definition — end-to-end encryption (E2EE): only your devices hold the keys to decrypt; the service provider can’t read the content even if it stores it.
Practical takeaway: Before enabling any AI coach or cloud feature, check what is processed on the device versus in the cloud, whether the provider uses data to improve models, and how to revoke sharing later.
“AI wearables” in everyday life — realistic use cases (and limits)
1) Perimenopause: understanding nights to improve days
A 49-year-old notices erratic sleep and daytime fatigue. Her ring shows increased nightly temperature variation and lower HRV during certain weeks; tagging symptoms helps correlate timing.
She tries behavioural changes (cooler room, earlier light exposure, consistent bedtime) and a cooling wrist device for disruptive night sweats. After a month, she sees improved sleep disturbance scores, echoing feasibility-study trends; she then discusses hormone therapy options with her clinician using the journaled data.
Evidence-based? The patterns and behavioural nudges, yes; the wrist cooling device has promising feasibility data, but larger randomised trials are pending.
2) Mental health: from metrics to action, without medicalising everyday stress
A mid-career manager uses a watch with EDA and a ring with HRV-based “stress” telemetry. After a month of baseline, the new AI coach recommends: one 20-minute afternoon walk, earlier dinner on late-meeting days, and a 10-minute pre-sleep wind-down using breath pacing.
On weeks with high travel, it automatically softens exercise targets. This aligns with behavioural science; still, LLM advice should cite sources, avoid diagnostics, and nudge users toward clinical care when screeners flag risk.
3) Sleep apnea suspicion: a watch nudges a clinic visit
A 42-year-old with loud snoring and daytime sleepiness receives a watch alert: multi-night data show signs consistent with moderate to severe OSA.
He books a telehealth consult and gets a formal home sleep test. That is the pathway these features are designed to encourage.
4) Atrial fibrillation: passive detection matters
An asymptomatic 66-year-old’s Fitbit flags an irregular rhythm pattern while sleeping; follow-up ECG confirms paroxysmal AFib. Early detection can lead to a stroke risk evaluation and discussion of treatment options. This is precisely the use case behind passive PPG algorithms cleared by the FDA.
What the evidence says (so far)
- AFib features (Apple/Withings ECG spot checks; Fitbit PPG background notifications) are supported by extensive validation datasets and established pathways. These are fundamental screening tools with documented performance bounds.
- Sleep apnea screening on consumer watches is authorised, accompanied by clear statements that it is not a substitute for a diagnosis. Clinical follow-up is required.
- Stress and mental-health detection is emerging: systematic reviews call it promising but not clinic-ready; consumer products should frame outputs as well-being guidance, not diagnoses.
- Menopause hot-flash devices, such as the Embr Wave, demonstrate feasibility and user-reported improvements (including sleep disturbance and perceived control), but large randomised trials are limited; buyers should calibrate their expectations accordingly.
- Cuffless blood pressure remains under verification; use cuff-based devices for medical decisions unless your clinician advises otherwise.
Risks and how to manage them
Standard failure modes: sensor bias, noisy data, over-medicalisation, and privacy leakage. Provide quick mitigations that readers can actually do this week.
Sensor bias and generalisation
Optical sensors can vary with skin pigmentation, tattoos, and perfusion. Regulators have pushed for inclusive validation; readers should prefer products with transparent testing and, if in doubt, spot-check with clinical devices.
Over-medicalisation and anxiety
Watches and rings can encourage what clinicians refer to as “data rumination.” Journalists raised this issue during the launch of Fitbit’s EDA-based stress features and Apple’s early ECG capabilities.
Practical mitigations: use weekly rather than daily goals, treat deviations as signals to observe, not as diagnoses, and lean on actionable behaviours (sleep regularity, daylight, steps).
Privacy and secondary use
End-to-end encryption for Health data (Apple) and “not used for ads” commitments (Google/Fitbit) are helpful, but read app screens carefully when enabling AI features — many ask for additional permissions to personalise. Adjust sharing settings periodically.
“Too good to be true” claims
The FDA warns against noninvasive glucose claims in unapproved smartwatches/rings; avoid products that promise clinical measurements without approvals or published evidence.
Practical buyer guidance (non-exhaustive)
Map goals to device classes and approved features. Keep it plain: what to buy for heart rhythm awareness, suspected OSA, stress coaching, menopause support, or metabolic insight.
If your goal is heart rhythm awareness, choose a watch with ECG and/or an FDA-cleared AFib feature (Apple Watch, Fitbit models with IRN/ECG; Withings ScanWatch). These are the most mature “medical” features in wearables.
If you suspect sleep apnea, a clinician-ordered home test remains the gold standard for diagnosing the condition. Consumer watch screening can be a helpful nudge, and in some regions, it is authorised to flag potential risks.
If stress awareness and coaching are your priorities, look for devices with EDA (such as Fitbit/Pixel Watch) or HRV-based resilience/stress features (like Oura/Whoop). Prefer platforms that clearly display how they calculate metrics and enable you to tag specific contexts (e.g., travel, illness, caffeine). Remember, these are wellness tools.
If you’re in perimenopause/menopause, any device with reliable nightly temperature trends and HRV helps with journaling and pattern spotting. Cooling wrist devices may also help with comfort, but their effectiveness remains under study.
If you want metabolic insights, consider whether an OTC CGM aligns with your goals and budget, and discuss it with a clinician if you have conditions or medications that affect glucose levels. Be cautious of non-invasive glucose claims on rings/watches.
Where the field is headed (2025–2028)
Expect tighter ring-watch-phone AI, on-device privacy by default, and more women ’s-health-first design. Regulation will demand prospective evidence—not demo reels.
- Tighter ring-watch-phone integration with AI summaries that explain why a metric changed and what the smallest practical action is (e.g., “10 minutes outdoor light after waking”).
- Condition-adjacent screening will expand beyond AFib and OSA as datasets grow (e.g., early illness detection from temp/HRV, atrial flutter variants), but regulators will demand prospective evidence.
- Women’s health will continue to diversify — beyond fertility to menopause, postpartum recovery, and pelvic-floor rehab — with more devices designed for women’s needs rather than adapted from sports trackers. Early signs are already visible.
- Privacy-preserving AI (on-device inference, Private Cloud Compute) will transition from a marketing focus to a necessity as people expect local processing by default.
- Equity by design: achieving accuracy across skin tones and body types will become a go-to-market requirement, not a nice-to-have, as the FDA and journals continue to pressure oximetry and PPG accuracy.
Bottom line
AI wearables in 2025 are most valuable when they help people do what we already know improves health — move more, sleep more regularly, manage heat and stress, and seek timely care. They are least valuable when they promise “diagnosis from a wrist” without evidence, or when they amplify anxiety and false certainty.
For menopause, mental health, sleep, and cardiometabolic risk, today’s best devices translate continuous signals into practical steps and clinical prompts.
The right question isn’t “Which device is smartest?” It is “Which one helps me change one small thing this week — and respects my privacy while doing it?”